by Marisa Wexler, MS | June 17, 2022
Treatment with the experimental immune-modulating therapy vidofludimus calcium reduced disease activity on MRI scans in adults with relapsing-remitting multiple sclerosis (RRMS), data from the Phase 2 EMPhASIS clinical trial shows.
Top-line results from EMPhASIS were reported by the therapy’s developer Immunic Therapeutics in 2020. Researchers at Immunic and other institutions now have published full trial results in the Annals of Clinical and Translational Neurology.
The study, “A double-blind, randomized, placebo-controlled phase 2 trial evaluating the selective dihydroorotate dehydrogenase inhibitor vidofludimus calcium in relapsing-remitting multiple sclerosis,” was funded by Immunic.
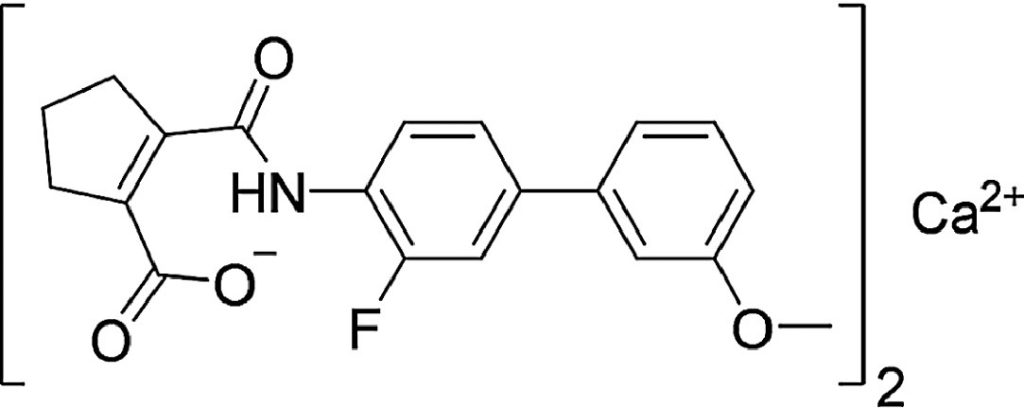
Vidofludimus calcium, also called IMU-838, is an oral therapy designed to reduce the activity of certain immune cells (B-cells and T-cells), thereby dampening the inflammatory attack that drives MS. Immunic is developing the therapy for MS and other inflammatory conditions, including inflammatory bowel disease and COVID-19.
The Phase 2 EMPhASIS clinical trial (NCT03846219) enrolled adults with RRMS, ages 18 to 55, at 38 sites in Bulgaria, Poland, Romania, and Ukraine. A total of 209 participants were assigned randomly to take vidofludimus calcium at one of two doses — 30 mg or 45 mg — or a placebo, every day for 24 weeks (about six months).
A total of 198 (95%) participants completed the study. Among participants on vidofludimus calcium who didn’t finish the study, the most common reasons for stopping were side effects.
The study’s main goal was to assess the effect on total active lesions — including new lesions, enlarging lesions, and lesions that showed evidence of ongoing inflammation.
Results showed that both doses of vidofludimus calcium significantly reduced active lesion counts compared to placebo. Specifically, the 45 mg dose reduced lesion counts by 62%, and the 30 mg dose by 70% — the researchers noted that lesion counts were generally comparable with both doses.
The reduction in lesion counts among patients given vidofludimus calcium was evident as early as six weeks after starting treatment, and remained low for the remainder of the 24-week study.
“The results from this phase 2 trial of vidofludimus calcium in patients with RRMS are encouraging, as the trial met its primary and key secondary endpoints for suppressing the number of combined unique active magnetic resonance imaging lesions,” Robert J. Fox, MD, said in a press release. Fox is a neurologist at the Mellen Center for Multiple Sclerosis in Cleveland and lead author of the study.
Relapse rates were generally lower among patients given vidofludimus calcium, but the difference from placebo was not statistically significant. Also of note, levels of neurofilament light chain (NfL), a marker of damage in the nervous system, increased over the 24-week study in patients given placebo. In contrast, average NfL levels decreased among participants on vidofludimus calcium.
“Trends favoring vidofludimus calcium were noted in clinical outcomes (i.e., annualized relapse rate) and clinically relevant biomarkers (i.e., neurofilament light chain), although the study was not [designed to test] these outcomes,” the researchers wrote.
“Coupled with the short study duration and relatively low sample size, drawing definitive conclusions on non-MRI outcomes in this context is limited. Longer studies in a larger patient population are needed to demonstrate the effect of vidofludimus calcium on clinical outcomes,” they added.
Vidofludimus calcium was generally well-tolerated in the EMPhASIS clinical trial. The proportion of patients who reported any adverse events (medication side effects or other safety-related events) was similar among participants given placebo or vidofludimus calcium at either dose — just over 40% in all three groups. The most common adverse events in all three groups were headache and a common cold. Most of these events were mild or moderate in severity.
No serious adverse events were considered related to treatment with vidofludimus calcium, and there were no deaths during the study.
“Importantly, vidofludimus calcium was found to be safe and well-tolerated as compared to placebo, with no increase in the rate of infections, effects on liver or blood cell laboratory parameters and with a very low treatment discontinuation rate,” said Fox.
Immunic is conducting two identically designed Phase 3 trials, (ENSURE-1, NCT05134441) and (ENSURE-2, NCT05201638), to further evaluate vidofludimus calcium in adults ages 18 to 55 with relapsing forms of MS, including RRMS and active secondary progressive MS.
The Phase 2 trial CALLIPER (NCT05054140) also is investigating the experimental therapy in people ages 18 to 65 with primary progressive MS and nonactive SPMS.
“Our phase 2 CALLIPER trial in progressive multiple sclerosis patients [aims] to further explore vidofludimus calcium’s neuroprotective potential, as exemplified by a slowing of brain atrophy and delay in disability worsening, which are often caused by axonal and neural damage,” said Daniel Vitt, PhD, CEO and president of Immunic.
All three trials are enrolling participants at sites in the U.S. and Europe. For more information, click on the trials’ NCT identifiers.